New insights into the growth of protein self-assembled structures
A recent study published in Nature Communications Biology has identified a novel growth pathway for pathological protein aggregates. This is a discovery that may give us a better understanding of the onset and development of diseases such as Alzheimer’s and Parkinson’s.
The research was conducted in the framework of a collaboration between the teams of Associate Professor Vito Foderà from Dept. of Pharmacy and Associate Professor Nikos S. Hatzakis from Dept. of Chemistry.
Protein aggregates in the form of the so-called amyloid structures are a hallmark of a number of devastating conditions, such as
Alzheimer's and Parkinson's diseases. In vitro analysis of protein aggregation processes often relies on bulk methods, providing limited information on the aggregation kinetics at the level of individual species.
The work, funded by the Lundbeck Foundation and titled “Direct observation of heterogeneous formation of amyloid spherulites in real-time by super-resolution microscopy", is published in Nature Communications Biology and reports a novel methodology for the analysis of amyloid systems, highlighting a pronounced heterogeneity of the aggregation mechanisms.
Vito Foderà explains further:
“By combining our expertise in protein aggregation and the microscopy-based technology developed by Assistant Professor Min Zhang in Nikos S. Hatzakis group we reported the first experimental observation of anisotropic growth of amyloid structures and the simultaneous quantitative evaluation of the thermodynamic parameters at the level of single aggregate.
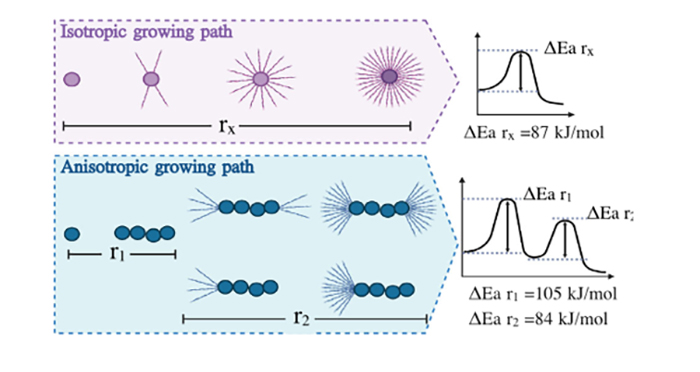
The growth of these self-assembled structures is not necessarily isotropic and may develop following specific directions in space. This mechanism is also observed for metal alloys, minerals and polymers, suggesting common physics laws ruling these processes. The ability to experimentally detect such a difference in the mechanism may help explaining the occurrence of the heterogeneous nature of the related diseases."
Vito Foderà, Nikos S. Hatzakis and Min Zhang are continuing their collaboration on the subject, focusing on protein systems in connection to Parkinson´s disease with the aim of identifying different mechanisms leading to different subsets of the disease.
